Biology lab report
Student’s name
Institutional affiliation
Date
BIOLOGY LAB REPORT
OSMOSIS AND DIFFUSION
Lab report代写 BIOLOGY LAB REPORT:PROCEDURE1.Get four 8 oz. cups and label each one with percentage concentrations of 0, 1.75%, 3.5% and 7% respectively.
PROCEDURE
1.Get four 8 oz. cups and label each one with percentage concentrations of 0, 1.75%, 3.5% and 7% respectively.
2.Measure 100 ml of distilled water and pour it into a fifth unlabeled cup
3.Measure out 1.5 level teaspoon of salt and add to the fifth cup with distilled water and mix. The resulting composition represents the 7% salt concentration solution.
4.Using a measuring cylinder, pour out a half (50 ml) of this solution into the cup bearing the label of 7%.
5.Top up the remaining 50 ml with distilled water to the 100 ml mark in order to half the salt concentration to 3.5%. Lab report代写**格式
6.Similarly pour out 50 ml of the resulting solution into the cup bearing the label of 3.5%.
7.Top up the remaining 50 ml 3.5% salt solution with distilled water to the 100 ml mark so as to dilute it to a concentration of 1.75%
8.Pour 50 ml of the resulting 1.75% into the cup bearing the appropriate label.
9.Empty the remaining solution and rinse the cup.
10.Using a sharp blade for best results, slice 8 identical pieces of potatoes to the dimensions (1 x 1 x 4) cm as precisely as possible.
11.Determine the volume of each potato core with the help of the mm ruler and the calipers. The volume of each strip as per the provided dimensions should be exactly 4 cubic centimeters.
12.Into each cup, place two measured cores for the duration of the night or a minimum of 8 hours.
13.Remove two cores from one cup, dry them with a paper towel, then measure and tabulate the average result in a table.
14.Repeat the above step for the remaining three cups.
RESULTS AND OBSERVATIONS Lab report代写
At the end of the experiment, the final potato core dimensions obtained were as follows;
| Width (cm) | Height (cm) | Length (cm) | Volume (cm3) | |
| 0% strip | 1.2 | 1.2 | 4.5 | 6.48 |
| 1,75% strip | 0.97 | 0.98 | 4.2 | 3.99252 |
| 3.5%strip | 0.8 | 0.82 | 3.7 | 2.4272 |
| 7% strip | 0.74 | 0.75 | 3.6 | 1.998 |
Questions on osmosis Lab report代写
1)Average percentage volume difference
Volume difference percentage = (volume difference / initial volume) * 100
0% solution volume difference percentage = ((6.48 – 4)/4) * 100 = 62%
1.75% solution volume difference percentage = ((4 – 3.99252) / 4) * 100 = 0.187%
3.5% solution volume difference percentage = ((4-2.4272) / 4) * 100 = 39.32%
7% solution volume difference percentage = ((4 – 1.998) / 4) * 100 = 50.05%
Tabulated results Lab report代写
| 0% SOLUTION (Cm3) | 1.75% SOLUTION (Cm3) | 3.5% SOLUTION (Cm3) | 7.0% SOLUTION (Cm3) | |
| INITIAL VOLUME | 4 | 4 | 4 | 4 |
| FINAL VOLUME | 6.48 | 3.99252 | 2.4272 | 1.998 |
| % DIFFERENCE | 62 | 0.187 | 39.32 | 50.05 |
2)Variables that may have affected the rates of osmosis Lab report代写
Temperature
Temperature generally increases the kinetic energy of molecules. As a result, the temperature conditions under which the experiment was conducted played a big role in determining how the results turned out.
Pressure conditions
The pressure difference that exists on either side of the semi-permeable membrane also weighs in on the rate of osmosis. This is because a higher pressure tends to push the water molecules across the semi permeable membrane to the opposite side.
Concentration gradient Lab report代写
A high concentration gradient results in a high rate of osmosis. Therefore, osmosis varied depending on the cell sap concentration of the specific potato used in the experiment. Different potatoes translate to different cell sap concentrations therefore different results despite similar initial dimensions.
3)
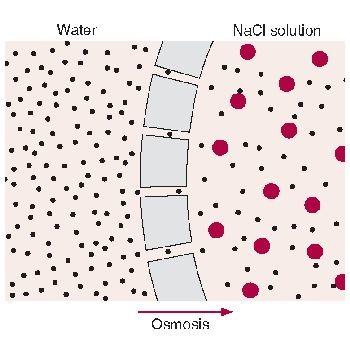
In osmosis, water molecules always move from a region of high solvent concentration (low solute) to a region of low solvent concentration (high solute). In other words, water molecules move from a dilute to a concentrated region.
4) Lab report代写
1. The freshwater organism is hypotonic to the salty water environment which is termed as being hypertonic to the organism. As a result, it will lose water though osmosis which will go on as long as there is a concentration gradient between the organism and its environment. The osmosis will only stop once the organism and the salt water become isotonic relative to one another, at which point, the organism will have shrunk in size and possibly died.
2.If a marine organism is placed in freshwater, the marine organism, being hypertonic to the freshwater, gains water through osmosis and swells. The osmosis continues until an isotonic equilibrium is achieved. In most cases, the swelling goes on till the organism bursts due to a lack of a cell wall.
5) Lab report代写
The addition of water to the carrots in the store served to maintain the cell turgidity of the carrots. The carrots take up water through osmosis causing them to not only to increase in weight, but in size as well. In this case, the water absorbed accounted for a total of 0.8 pound of the initial carrot weight.
6)
Pouring salt on leeches effectively creates a hypertonic environment about its body. In an aim to achieve osmotic balance (isotonic situation), the leech will lose water though osmosis causing it to shrink and fall off.
1. Illustration of the importance of Surface-to-volume Ratios Lab report代写
a)Calculate the surface-to-volume ratio of the following potato cubes
I. CUBE 1 : Length, width and height are all 5 mm
S.A to V ratio = S.A/V
In this case, S.A = 5 * 5 * 6 = 150 mm3
Volume = 5 * 5 * 5 = 125 mm3
S.A to V ratio = 150/25 = 0.01198
II. CUBE 2 : Length, width and height are all 3 mm
S.A to V ratio = S.A/V
In this case, S.A = 3 * 3 * 6 = 54 mm3
Volume = 3 * 3 * 3 = 27 mm3
S.A to V ratio = 54/27 = 2
b)Effect of cell size on diffusion rate Lab report代写
Procedure
1. Peel a potato
2.From the peeled potato, cut two cubes of dimensions (1 x 1 x 1) cm
3.Cut another two cubes of dimensions (1.5 x 1.5 x 1.5) cm
4.Further cut another pair of potato strips of dimensions (2 x 2 x 2) cm
5.Pour distilled water into a cup and add some food coloring till a dark color is achieved.
6.Place the cubes into the solution ensuring that they are fully submerged for duration of 2 – 4 hours. Lab report代写**格式
7.After the duration, remove the cubes from the solution and accurately cut each cube in half and measure how far the solution has diffused into the potato cube.
8.Tabulate your findings
9.Calculate the various rates of diffusion keeping in mind that;
Rate of diffusion (cm/min) = distance of diffusion/time
Results and observation Lab report代写
| DISTANCE (cm) | AVERAGE DISTANCE (cm) | |
| 1 cm cube | 0.4 | 0.4 |
| 1 cm cube | 0.4 | |
| 1.5 cm cube | 0.35 | 0.335 |
| 1.5 cm cube | 0.32 | |
| 2 cm cube | 0.22 | 0.235 |
| 2 cm cube | 0.25 |
Diffusion questions Lab report代写
7)Calculations
1 cm cube surface to volume ratio = 6/1 = 6
1 cm cube rate of diffusion = distance of diffusion/time
= 0.4 / 120
=0.00333 cm / min
1.5 cm cube surface to volume ratio = 13.5 / 3.375 = 4
1.5 cm cube rate of diffusion = distance of diffusion/time
= 0.335 / 120
=0.00279166 cm / min
2 cm cube surface to volume ratio = 24 / 8 = 3
2 cm cube rate of diffusion = distance of diffusion/time
= 0.235 / 120
=0.001958333 cm / min
Analysis and answers Lab report代写
8)The (1 x1 x 1) cm cube would be the most effective cube in transporting materials in and out of the cube. This is because it is the one with the highest calculated diffusion rate because it has the highest surface area to volume ratio among the cut potato pieces.
9)As observed in the experiment, diffusion rates and cell sizes are inversely proportional. This is to say that the larger the size of a cell, the slower the rate of diffusion and vice versa. As a result, efficient diffusion requires smaller cell sizes and this efficiency reduces as the cell sizes increase. Lab report代写**格式
10)The quarter pound meatball will cook at a faster rate relative to that of the quarter pound hamburger. The reason behind this observation is that the meatball, being more compact, has a larger surface to volume ratio hence will allow heat to diffuse at a faster rate than the hamburger.
11)The ears of hares in hot environments are adapted to expose a large surface area over which excessive heat from the body is lost. Were the ears of these hares not elongated, then the surface to volume ratio would be reduced hence the efficiency of heat loss though diffusion would be compromised.
更多其他: Report代写 Case study代写 Proposal代写 Capstone Projects Essay代写 数据分析代写 Review代写 研究论文代写 文学论文代写 Academic代写 商科论文代写 论文代写



您必须登录才能发表评论。